Owkin, a Franco-American start-up specializing in AI and federated learning applied to medical research, announced on September 2 that two of its diagnostic solutions, namely Owkin Dx RlapsRisk BC and Owkin Dx MSIntuit CRC, have received CE-IVD (CE Marking for In Vitro Diagnostic) certification, which will allow it to market these solutions in Europe and thus improve the management of patients suffering from breast or colorectal cancer.
Co-founded in 2016 by Thomas Clozel, a clinical researcher and former assistant professor of clinical hematology, and Gilles Wainrib, a PhD in AI applied to biology, Owkin is based in the United States, but the majority of its team is based in Paris. Its mission is to use AI to discover and develop better treatments for unmet medical needs, starting with the fight against cancer.
Meriem Sefta, Director of Diagnostics at Owkin, states:
“Our mission is to use AI to find the right treatment for each patient. New drugs allow us to personalize treatments based on patients’ individual disease characteristics, promising a new era of precision medicine. But one of the hurdles doctors face is finding those patients quickly, accurately and efficiently.”
She adds of the Owkin Dx RlapsRiskBC and Owkin Dx MSIntuit CRC solutions:
“Our first approved diagnostic solutions could help millions of breast and colorectal cancer patients get the treatment they need earlier. This is a pivotal moment in Owkin’s mission to use AI to better understand diseases and develop more accurate diagnostics and treatments for patients.”
The RlapsRisk BC solution
RlapsRisk BC is an AI-based prognostic solution designed to predict the likelihood of an early breast cancer patient (ER+/HER2-) relapsing after treatment, allowing oncologists to determine which high-risk patients can benefit from targeted therapies and which low-risk patients can potentially avoid chemotherapy.
It was developed in collaboration with Institut Gustave Roussy, Europe’s leading cancer center, after Owkin won the 2019 “AI for Health” challenge from the Île de France region.
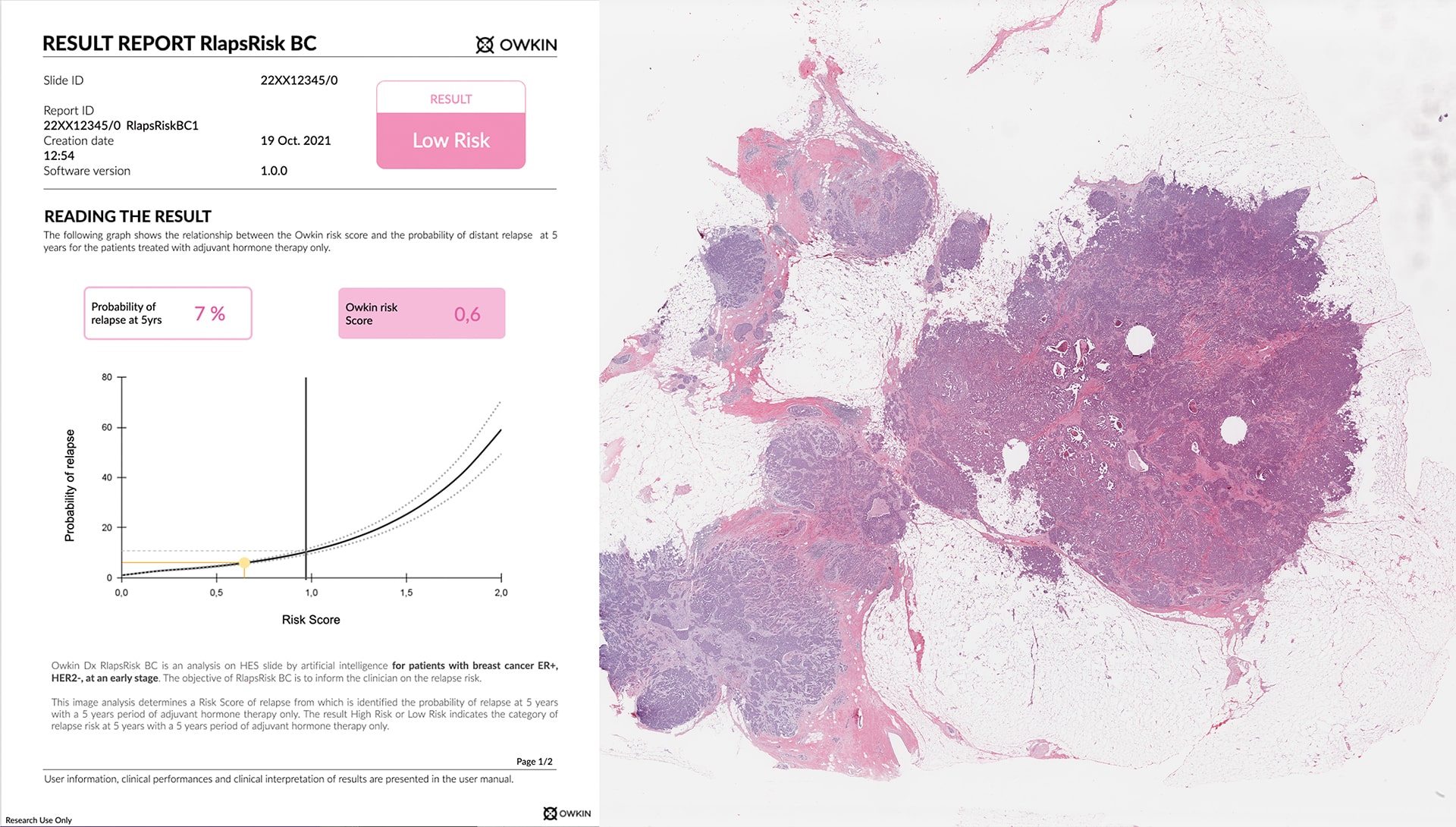
Assessing the risk of relapse, using AI, after treatment from digital images of tissue samples will truly change breast cancer care management. Currently, breast cancer patients at risk of relapse are identified through costly and time-consuming molecular or genetic testing. RlapsRisk BC offers performance comparable to standard molecular tests, while using much more readily available data, reducing turnaround times (about 15 minutes) and democratizing access to precision medicine.
Dr. Magali Lacroix-Triki, MD, PhD, a pulmonary specialist in the Department of Medical Biology and Pathology at Institut Gustave Roussy, states:
“AI-based digital pathology diagnostics could help us provide a comprehensive analysis of each tumor from a single representative standard-stained tumor slide, in a complementary process to the pathologist’s diagnosis. This would democratize access to precision medicine, opening a new era of treatment for patients worldwide.”
MSIntuit CRC is a digital pathology AI diagnostic solution that screens for a biomarker in colorectal cancer tumors known as microsatellite instability (MSI), a defect in a cell’s ability to correct errors that occur when DNA is copied. This diagnosis excludes microsatellite stable phenotypes (MSS), allowing pathologists to focus their resources on confirming patients with MSI.
Based on deep learning, it was trained on hundreds of patient samples until it could accurately detect the presence of MSI in tumor images. Its accuracy was validated by a blinded clinical validation study on hundreds of additional patient samples.
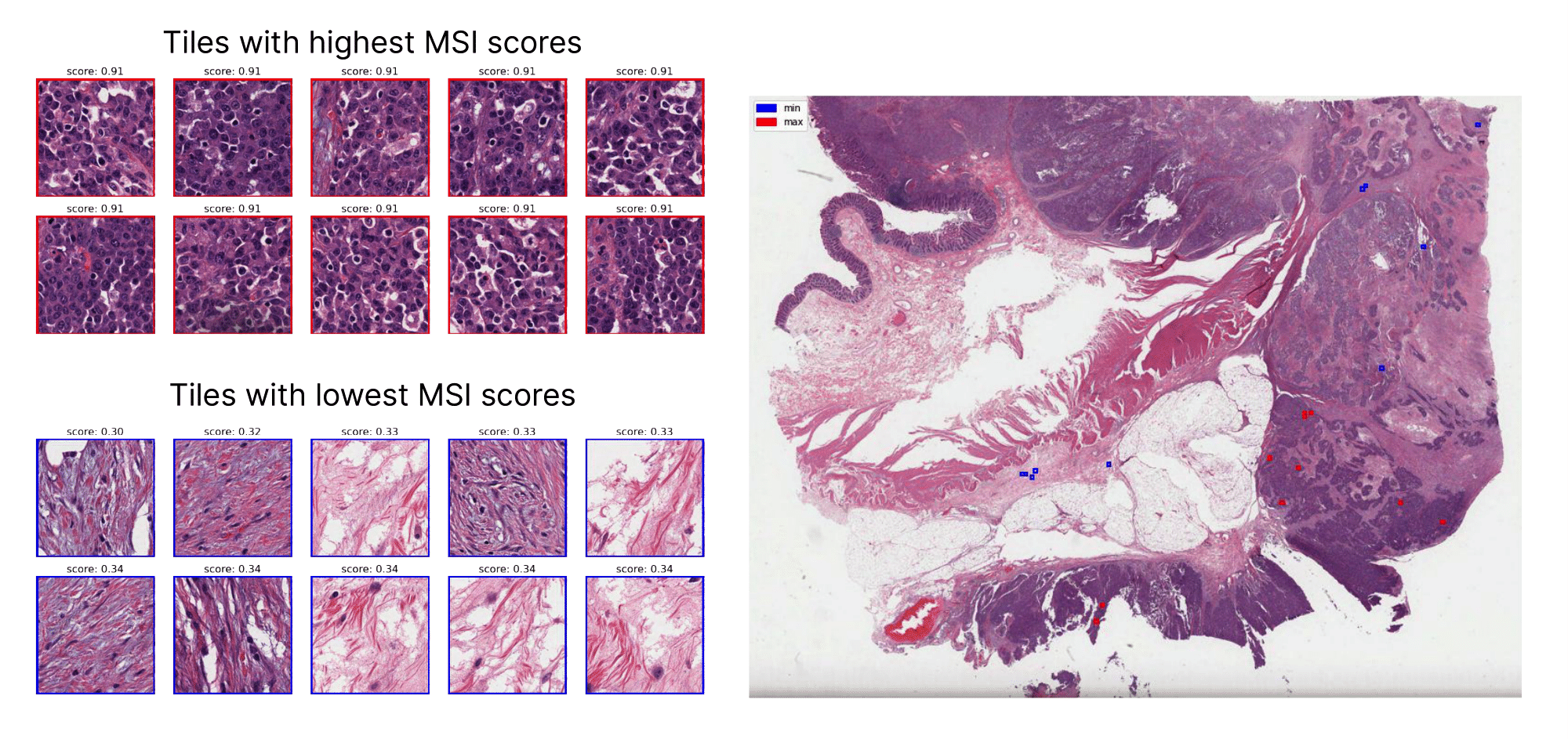
It allows physicians to screen patients more efficiently than with standard testing techniques, which include time-consuming polymerase chain reaction (PCR) or immunohistochemistry tests and patient tissue samples.
In addition, patients with high-MSI tumors are eligible for treatment with the new immunotherapy drug pembrolizumab, which, according to a 2020 clinical trial, led to significantly longer progression-free survival than standard therapy when received as first-line treatment for metastatic MSI-H-dMMR colorectal cancer.
Professor Magali Svrcek, MD, PhD, Sorbonne University, AP-HP, Hôpital Saint-Antoine, Department of Pathology, Paris, said:
“As colorectal cancer is the third most common cancer in the world, it is crucial that we are able to screen patients for biomarkers that make them eligible for potentially life-saving treatments. dMMR/MSI screening should be performed on all colorectal cancer patients, but pathologists’ time and resources are often overstretched.”
She concludes:
“Owkin’s new AI diagnostic solution, MSIntuit CRC, will account for about half of the number of cases requiring screening. It is designed to help more patients receive treatment earlier. AI-based digital pathology algorithms like these, developed in consultation with pathologists, will soon be an integral part of our daily practice. They will help patients get the best treatment for them sooner – a new era of personalized medicine.”
Translated from IA et cancer : OWKIN présente deux solutions de diagnostic basées sur l’IA ayant reçu la certification CE-IVD