Clinical trials are fundamental for researchers to test the efficacy and tolerance of patients to a treatment. The selection of people for these trials must therefore be strict but inclusive, which remains complex. A team of researchers from Stanford University, in collaboration with the biotech company Genentech, has developed a system based on artificial intelligence that can safely group together participants for a clinical trial who might have been excluded in the past, such as pregnant women.
In a publication in Nature, Ruishan Liu, Shemra Rizzo, Samuel Whipple, Navdeep Pal, Arturo Lopez Pineda, Michael Lu, Brandon Arnieri, Ying Lu, William Capra, Ryan Copping and James Zou, researchers at Stanford University and Genentech, presented Trial Pathdfinder. Their solution enables the systematic analysis of the effect of different eligibility criteria on cancer trial populations and outcomes with real-world data using the Trial Pathfinder computational framework. The scientists explained in the article:
“We apply Trial Pathfinder to emulate completed lung cancer trials (not with advanced small cells) using data from a national electronic health record database with 61094 such lung cancer patients.
Our analyses reveal that many common criteria, including exclusions based on multiple laboratory values, had minimal effect on the risk ratios of the trials. When we used a data-driven approach to broaden the restrictive criteria, the pool of eligible patients more than doubled on average and the relative risk of overall survival decreased by an average of 0.05. This suggests that many patients who were not eligible under the original trial criteria could potentially benefit from the treatments.
We also support our results with analyses of other cancer types and patient safety data from various clinical trials. Our data-driven methodology for assessing eligibility criteria can facilitate the design of more inclusive trials while maintaining safeguards for patient safety.”
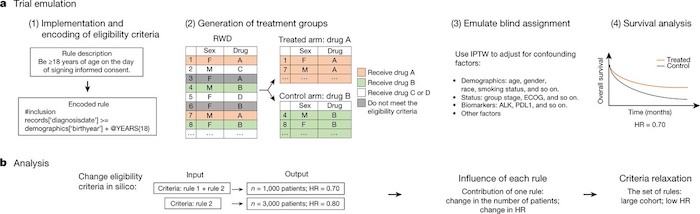
This tool should therefore make it possible to refine the selection criteria according to the type of research and to include the widest possible range of populations in clinical trials. According to the researchers, the use of Trial Pathdfinder would increase the number of people who could potentially participate in clinical trials by about 53%.
Translated from L’Université de Stanford et Genentech ont présenté Trial Pathdfinder, un système d’aide à la réalisation d’essais cliniques









