On February 24, Sanofi announced that it had joined forces with Medical Intelligence Service (MIS) to develop AccelRare, an Artificial Intelligence solution designed to reduce the time spent by patients suffering from rare diseases. This solution, which is based on MedVir, a pre-diagnosis tool from MIS, aims to accelerate the pre-diagnosis of 270 rare diseases that have an appropriate treatment or management. At the end of its development, AccelRare will be made available to general practitioners, pediatricians and specialists on the Internet. The tool should be available by the end of 2022, following tests carried out by accredited expert centers.
There are nearly 7,000 rare diseases identified to date, and one person in 20 is affected, i.e. three million French people. Half of the patients suffering from one of these diseases have no precise diagnosis. In France, the average time to diagnosis is 2 to 3 years, but 25% of patients remain without a diagnosis for 5 to 15 years. Rare diseases affect 350 million people worldwide.
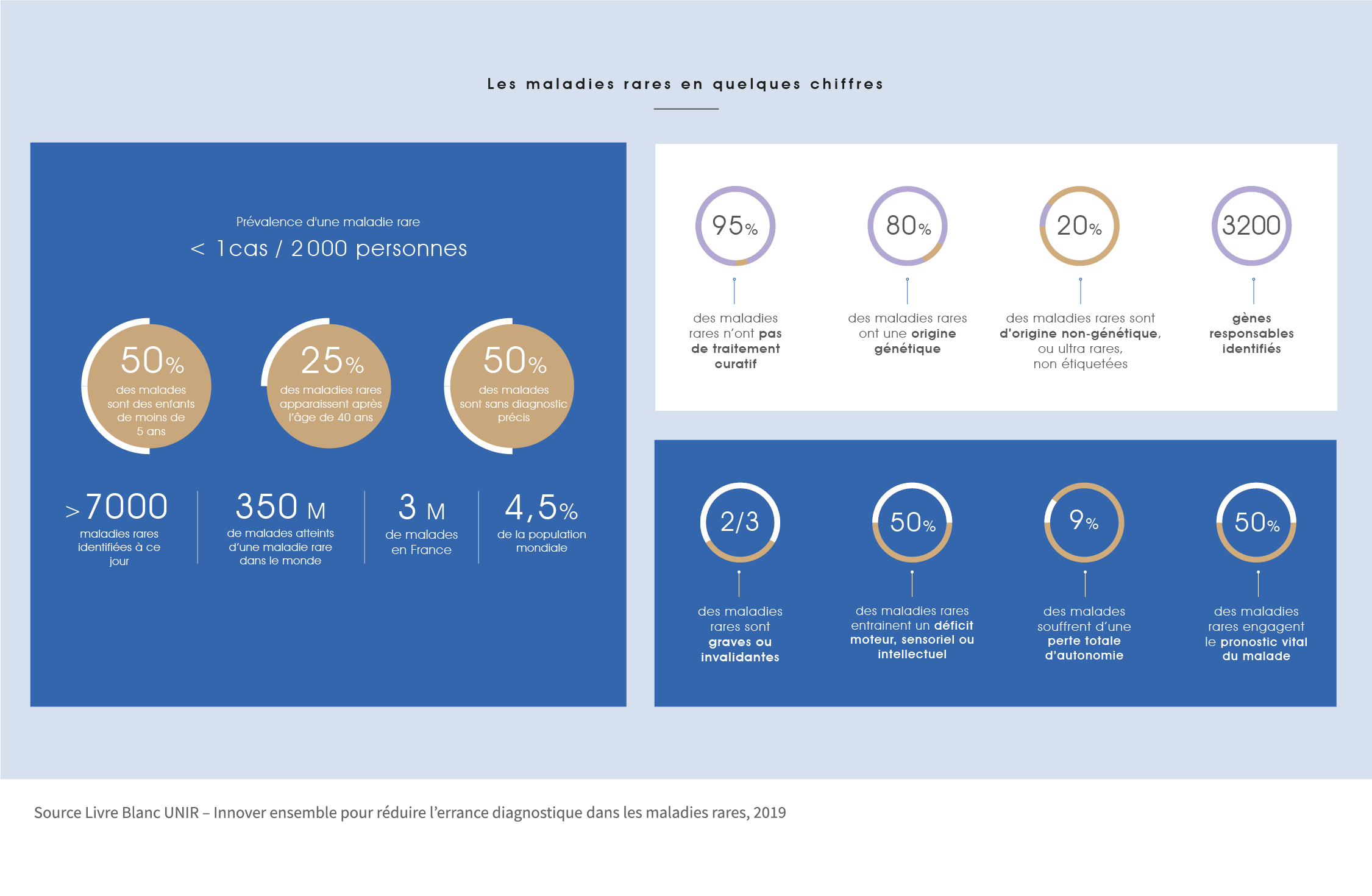
The majority of rare diseases are of genetic origin, some are known such as cystic fibrosis, Huntington’s disease… some autoimmune or auto-inflammatory diseases are included. They are called orphan diseases if no treatment is available to fight them.
Rare diseases in France
France set up the first national plan for rare diseases in 2005 (PNMR) and initiated a sustained dynamic of public action in favor of the fight against these pathologies on a European scale. In 2018, there were 387 national rare disease reference centers (CRMR) accredited within French university hospitals, as well as 175 identified regional centers of expertise. All rare diseases are severe, chronic and progressive. Early treatment, truly adapted to the disease, not only improves the quality of life of patients but also allows them to benefit from medical and social support. Making a diagnosis is therefore vital for patients, reducing the time between the first symptoms and diagnosis is a priority of the 3rd National Plan for Rare Diseases (2018-2022).
A participatory approach has been launched, with the aim of identifying innovative technological solutions that can provide answers to this challenge: UniR, whose white paper, published in December 2018, as part of this 3rd PNMR, brings together several proposals resulting from the reflection of Sanofi teams and a collective of around forty players from the rare disease community: Rare Disease Health Networks (Filières de Santé Maladies Rares – FSMR), expert and non-expert healthcare professionals, patient associations, digital startups, Foundations, Think Tank.
AccelRare
AccelRare is a solution that meets the 3 challenges of UniR:
– To improve the identification of atypical clinical pictures;
– Helping to coordinate players and refer patients;
– Facilitate the sharing of national and international expertise.
AccelRare is based on the MedVir pre-diagnosis tool proposed by MIS, the result of more than thirty years of French medical research led by Dr. Loic Etienne, a pioneering emergency physician. This decision support tool for regulation, orientation, diagnostic assistance, prevention and understanding of medicine was developed from a unique database and an AI algorithm by MIS and is distributed by Sanofi.
A very simple solution to use
During a consultation, if a patient presents symptoms and atypical clinical signs, the doctor enters these into AccelCare. The algorithm will identify if there is a significant risk of rare disease(s). It then provides a list of rare diseases associated with the symptoms described, information on these diseases and the contact information of the nearest accredited expert center. Currently, only the 270 rare diseases for which a treatment exists are concerned but the objective is to pre-diagnose the 7000 known diseases.
At the end of the consultation, the doctor decides whether or not to send the patient to the labelled expert center adapted to the patient’s situation and authorised to confirm this pre-diagnosis. If the disease is confirmed, the center will organize the patient’s therapeutic, paramedical and social care, as well as regular monitoring, with the aim of slowing down the progression of the disease and improving quality of life.
Deployment of the solution
In 2022, Sanofi’s Digital Innovation and Medical teams will test AccelRare with rare disease experts, based on real clinical cases and the list of 270 rare diseases for which a treatment has been found, to ensure that the solution makes the right pre-diagnosis. AccelRare will then be available on the Internet for all healthcare professionals, with a lexicon of symptoms adapted to the vocabulary of patients, in French and English, and later in other languages.
Translated from Sanofi et MIS s’associent pour diagnostiquer les maladies rares grâce à l’Intelligence Artificielle